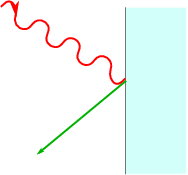
When we shine a lamp on a metal surface, electrons escape the surface. This is a simple experimental fact, that can easily be demonstrated. The intreaguing point is that it takes a minimum frequency of light to remove electrons from a metal, and different metals require different minimal frequencies. Intensity plays no rôle in the threshold.
The explanation for this effect is due to Einstein (actually he got the Nobel price for this work, since his work on relativity was too controversial). Suppose once again that light is made up from photons. Assume further that the electrons are bound to the metal by an energy {E}_{B}. Since they need to absorb light to gain enough energy to escape from the metal, and it is extremely unlikely that they absorb multiple photons, the individual photons must satisfy
|
hν > {E}_{B}.
| (1.5) |
Thus the minimal frequency is {ν}_{0} = {E}_{B}∕h. This is based on the quantum nature of light.